
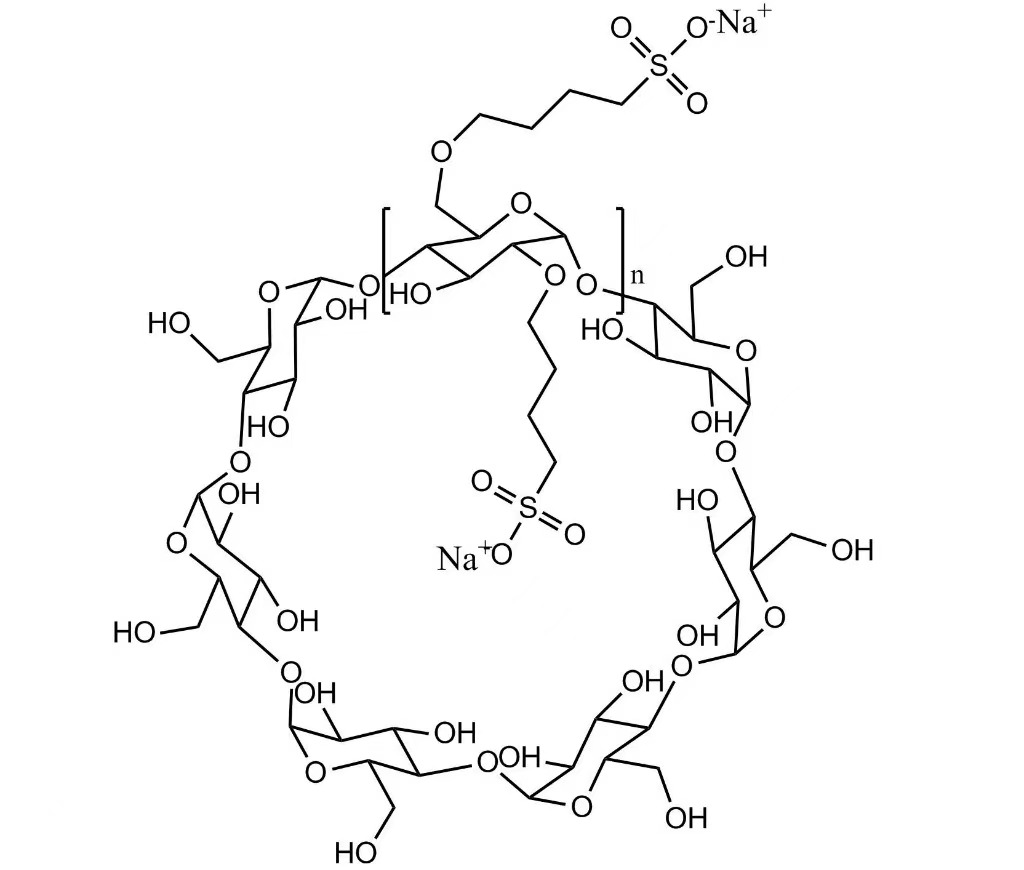
Sulfobutyl Beta Cyclodextrin Sodium CAS NO 182410-00-0
CAS No.: 182410-00-0
Formula hypothetica: C42H70-nO35·(C4H8SO3Na)n
Gradus: Iniectio Grade
Executive Standard: USP / EP / Enterprise Standard
Area application: Pharmaceutical Usus
Packaging: 500 g/peram; 1 kg/bag; 10 kg/pera vel tympanum; nativus packaging praesto
Sulfobutyl Ether Beta Cyclodextrin Sodium (Iniectio Grade)est summus perficientur cyclodextrin anionica producta inde aXi'an DELI Biochemical Industry Co., Ltd.. Ex Galeno pharmaceutico late adhibetur ad solutionem medicamentorum, stabilitatem, stabilitatem, ad emendandas varias dosis formas.
Hoc productum forms firmumnon-covalent inclusione complexorumcum activo medicamentis pharmaceuticis, praesertim nitrogenio-continentibus medicamentis, adiuvando ad augendam bioavailbilitatem, toxicitatem minuendam, larva odoris aut saporis injucundi.
Sulfobutyl Ether Beta Sodium Cyclodextrin in injectivis, oralibus, nasi et ophthalmicis formulis feliciter adhibitum est et ut tutius jocus cognoscitur derivationibus beta-cyclodextrin traditis.
Product nomen:Sulfobutyl Ether Beta Cyclodextrin Sodium
CAS No.:182410-00-0
Formulae hypotheticae:C42H70-nO35·(C4H8SO3Na)n
Gradus:Iniectio Grade
Executive Standard:USP / EP / Enterprise Standard
Area applicationis:Usus pharmaceuticus
Packaging:500 g/peram; 1 kg/bag; 10 kg/pera vel tympanum; nativus packaging praesto
|
Descriptio et Solubilitas/ videre singula in USP Monograph |
Albus ut off-album, in aqua solutum, in aqua parce solutum, in methanolo parce solutum, in ethanolo, in n-hexane, in 1-butanolo, in acetonitrile, in 2-propanolo, et in ethylacetate. |
|
sativum A/IR; USP<197K> |
Prosequitur SBECD reference |
|
Lepidium sativum B/(Assay modum)HPLC; USP<621> |
tR maioris cacuminis prosequitur SBECD reference |
|
Lepidium sativum C/CE; USP<1053> |
Requisitis experimenti convenit Mediocris Substitutionis gradus. |
|
Lepidium sativum D/USP<191> |
Positivum tium sodium |
|
Asssay/HPLC;USP<621> |
95.0%-105.0% in anhydrous basis |
|
Limit of Beta Cyclodextrin(Betadex)/IC; USP<621>;USP<1065> |
NMT 0.1% |
|
Limit of 1,4-Butane Sultone /GC;USP<621>, |
NMT 0.5ppm |
|
Limit of Sodium chloridi /IC; USP<621>;USP<1065> |
NMT 0.2% |
|
Limit of 4-acidi hydroxybutanei-sulfonici /IC;USP<621>;USP<1065> |
NMT 0.09% |
|
Limit of Bis(4-sulfobutyl) aetheris disodium /IC;USP<621>;USP<1065> |
NMT 0.05% |
|
Bacterial Endotoxins Test/USP<85> |
≤10EU/g |
|
Microbial Enumeratio Probat/USP<61> |
TAMC≤100cfu/g* TYMC≤50 cfu/g |
|
Expertus de certis Microorganismi/USP<62> |
Absentia Escherichia Coli/1g |
|
Solutio claritas (30%, w/v) / visual; vide singula in USP Monograph |
Solutio patet, et per se immunis a particulis materiae exterae. |
|
Mediocris gradus substitutionis [DS]/CE;USP<1053> |
6.2-6.9 |
Quisque batch of *Sulfobutyl Ether Beta Cyclodextrin Sodium (Iniectio Grade)fit completaTestimonium Analysis (COA).
COA speciem, pugionem, primordium, immunditiam limites, bacteria endotoxinos, limites microbiales et medium substitutionis gradum secundum USP requisita comprehendit.
COA et documenta technica pertinentia praesto sunt roganti pro qualitate recensionis et regulatorii referentiae.

1. Praeclara aquae solutio ad iniectionem formulae idoneae.
2. Formae inclusio stabilium complexorum cum amplis medicamentis pharmaceuticis activis.
3. Improves solubilitatem et bioavailability medicamentorum male solutorum.
4. Toxicitatem renum reducet et effectus hemolyticos minuit.
5. Adjuvat medicamentum emissio temperantiae rate et larvae ingratus odoris aut gustus.
6. Iniectio gradus qualitas obtemperans USP signis.
Xi'an DELI Industry Biochemical Co., Ltd. anno 1999 conditum est et in investigatione, evolutione et productione cyclodextrinum eiusque derivatorum plus quam XXVI annos intendit.
Societas speciales in excipientibus pharmaceuticis et commeatus cyclodextrin-substructis productis pharmaceuticis, veterinariis, et chemicis applicationibus ad clientes globali.
Cum processibus productionis stabilis et de administratione qualitate stricta, Xi'an DELI Industrii Biochemici Co., Ltd. committitur ut certos fructus et diuturnum cooperationem provideat.

Xi'an DELI Industry Biochemical Co., Ltd. plus XX annos experientiae habet in investigatione, progressione et productione cyclodextrin-basis exceptae pharmaceuticae. Societas spectat ad excipientes summus qualitas, certa, et facilis, ut sociis pharmaceuticis et formulis globalibus.
Productio processus severitatis qualitatis temperantia in singulis gradibus constituitur, a rudi materia ad emissionem operis perfecti. Quaelibet batch sub ratione qualitatis bene definitae administrandi ratio conficitur et cum documentis integris, incluso certificatorio Analyseos (COA) et pervestigationis monumentorum, conficitur.
Sodium sulfobutyl aethereum beta-cyclodextrinum comitatus producitur ad normam USP requisitorum et late in usu parenteral, oral, et aliae formulae pharmaceuticae. Constancia stabilis ad batch-ad-batch certam in medicamento solutionis, stabiliizationis et formulationis progressionem praestat.
Cum dedicato technico manipulo, Xi'an DELI Industrii Biochemical Co., Ltd. auxilium technicum technicum praebet, regimen formulationis et documenta regulatoria subsidia ad applicationes speciales et adnotationes necessitates occurrentes.

Q1: Quod est principale munus Sulfobutyl Ether Beta Cyclodextrin Sodium?
Solubilizer et perplexus maxime usus est ad solutionem medicamentorum, stabilitatem, et incolumitatem in formulis pharmaceuticis.
Q2: Hoc productum est aptum ad formulas injectabiles?
Ita, iniectio gradus est et USP requisitis ad usum parentalem obtemperat.
Q3: An productum forma complexa medicamentis covalentes?
Imo, complexiones non covalentes stabilis cum moleculis medicamentorum format.
Q4: Potestne haec excepta toxicitas medicamento minuere?
Ita, adiuvare potest ad toxicitatem renum et hemolyticos effectus reducendos cum quibusdam solubiliariis traditis.
Q5: Quae forma dosis hoc productum uti potest?
Convenit injectabiles, orales, nasi et ophthalmicae formas dosis.
Q6: technica auxilium praesto est?
Ita technica subsidia et adhibita moderatio postulantibus praeberi possunt.
Q7: Quid packaging magnitudinum praesto sunt?
Standard packaging comprehendit 500 g, 1 kg, et 10 kg. Customized packaging is available.
Q8: Quid est fasciae vita producti?
Pluteae vita est XXXVI mensium, cum consignata et propriis conditionibus reponuntur.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0