
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 est cyclodextrinum derivativum et novum Sinae DELI genus excipientium pharmaceuticae.
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 est novum Sinae DELI genus derivationum cyclodextrinum anionicarum altum solutum. Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 est sal sodium productum cum Betadex alkylated per 1,4-butanesulfonicum acidum lactonum sub conditionibus alkalinis. Est anion, Betadex summe aquarum solutum derivativum. Solubilizer, udus agente, chelante agente (agente multiplici) et agente larvante polyvatente, adhiberi potest.


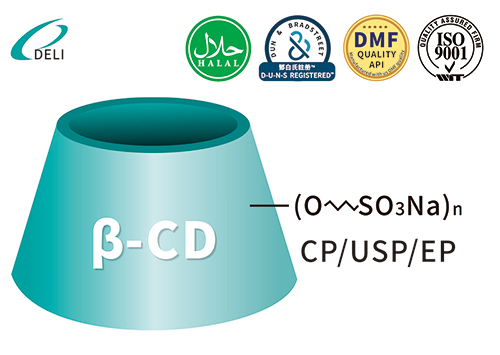 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0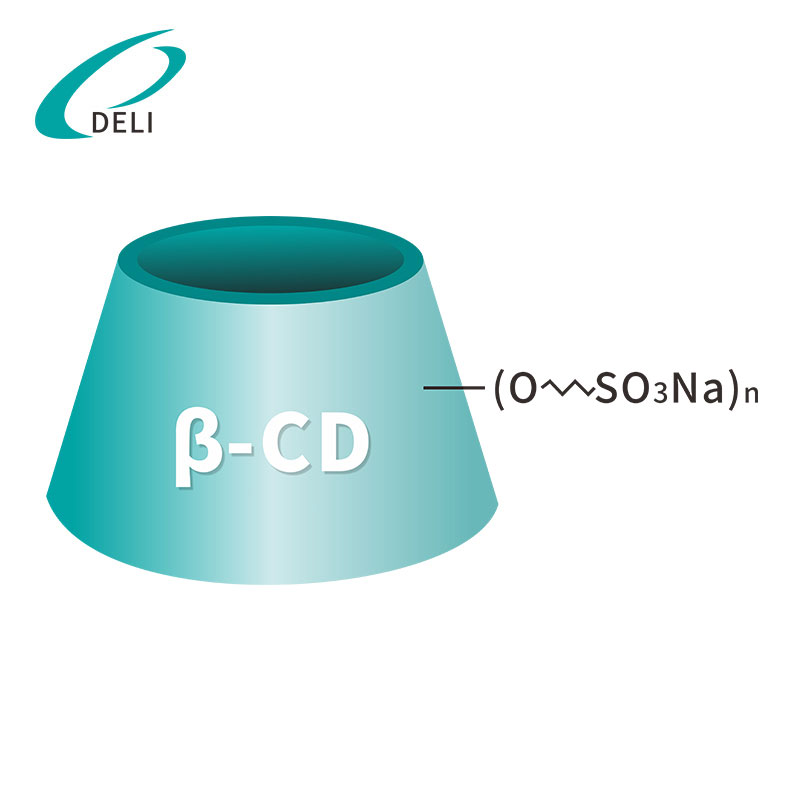 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium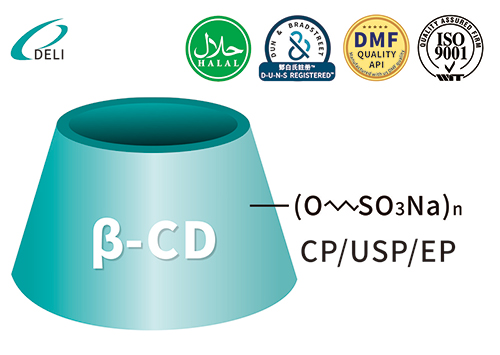 Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade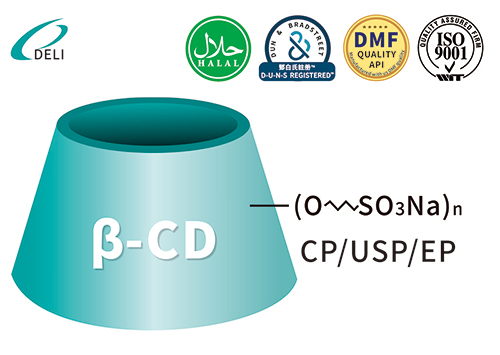 Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0 Sulfobutyl beta cyclodextrin 182410-00-0
Sulfobutyl beta cyclodextrin 182410-00-0