
Betadex Sulfobutyl Ether Sodium Injectabile Pharmaceuticum Excipiens EP USP Gradus (CAS 182410-00-0) est cyclodextrinum solutum valde derivativum adhibitum ut solubilizer et stabiliens in injectabili formularum medicamentorum, solutionem, salutem et bioavailbilitatem amplificandi.
Xi'an Deli Biochemical Industry Co., Ltd.summus technicus inceptio specialis est in investigationibus, evolutionibus, productionibus et venditionibus derivationum cyclodextrinum. Statuta anno 1999, societas plus quam 25 annos experientiae habet in excipientibus pharmaceuticis, inclusisHydroxypropyl Betadex (HPBCD)etBetadex Sulfobutyl Ether Sodium (SBECD). Societas mundanis areis dedicatas operatur et lineas productiones provexit, summus qualitas stabilis et constantis fructus pro applicationibus pharmaceuticis globalibus operatur.
Betadex Sulfobutyl Ether Sodium Injectable Pharmaceutical Excipient EP USP Grade(CAS 182410-00-0) est cyclodextrinum derivativum anionicum anonicum valde aquaticum. SBECD functiones quasi solubilizer, udus agentis, agentis fraudantis et larvatum agentis, pharmacum solubilitatem, stabilitatem, ac salutem in injectabilibus, oralibus, nasi et ophthalmicis formulae augendae. SBECD late in APIs antifungalis, antiviralibus, cardiovascularibus et oncologia usurpatur.

CAS No: 182410-00-0
Formula hypothetica: C42H70-nO35·(C4H8SO3Na)n
Gradus: Injectable, EP et USP obsequentem
Aspectus: Alba ad extemporalitatem amorpho pulveris
Dic: ≥99.0%
Applications: Injectabiles et parentales formulae pharmaceuticae
Packaging: 500g/peram; 1kg/bag; 10kg/ pera; 10kg/orum; nativus packaging praesto
Repono: Signatum et siccum
Vita fasciae: 36 months
COA / Quality Assurance:
Quaelibet massa SBECD probata est ad temptandam puritatem, endotoxin bacterial, menstrua residua, metalla gravia et mediocris gradus substitutionis (DS), cum USP et EP signis pharmacopticis obtemperans.
Betadex Sulfobutyl Ether Sodium adhibetur ad solutionem augendam, toxicitatem renum minuendum, hemolysin minuendam, et obsequium patientem emendandum. SBECD convenit systematibus intravenosis, intramuscularibus, nasalibus, oralibus et ophthalmicis partus. Complexiones inclusionem format cum APIs basica et nitrogen-continente aegre, stabilitatem emissionem sustentatam et meliorem stabilitatem praebens.
Hydroxypropyl Betadex (HPBCD) - Injectabilis et oralis pharmaceutica excipiens
Betadex Sulfobutyl Ether Sodium (SBECD) - Injectable-gradus cyclodextrinum derivativum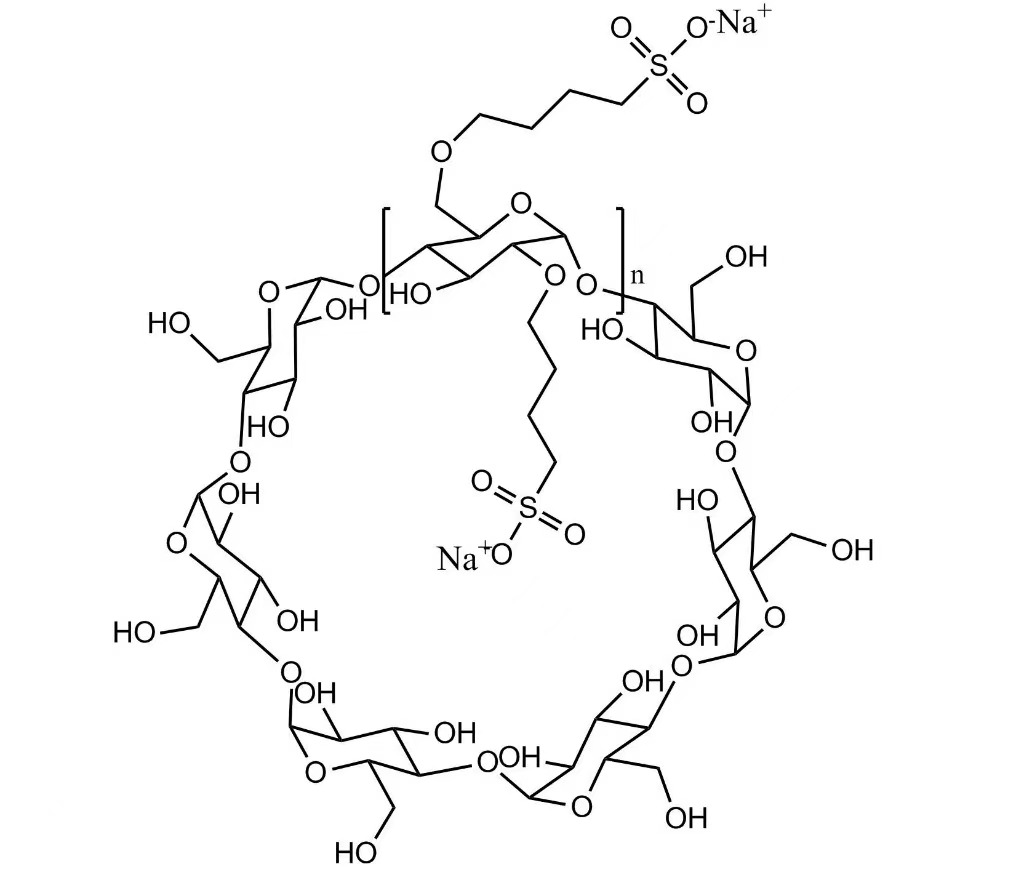
Q: est Xi'an Deli Biochemical fabricam SBECD?
A: Ita, Xi'an Deli Biochemical efficit Betadex Sulfobutyl Ether Sodium (SBECD) et HPBCD. Beta Cyclodextrin usus est ut materia rudis productionis derivativae.
Q: An exempla praesto?
A: Ita, SBECD exemplaria aestimationis praesto sunt.
Q: Quid est massae et annui productionis capacitas?
A: SBECD batch productionis capacitas est 2.5 talenta, et annuum output excedit 200 talentorum.
Q: Quae documenta regulatory provisa sunt?
A: COA, MSDS, notitia stabilitatis, et dossarii technici omnibus batches praesto sunt.
Q: Shipping optiones?
A: Global shipping by air, sea, or express tabellarium.


 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade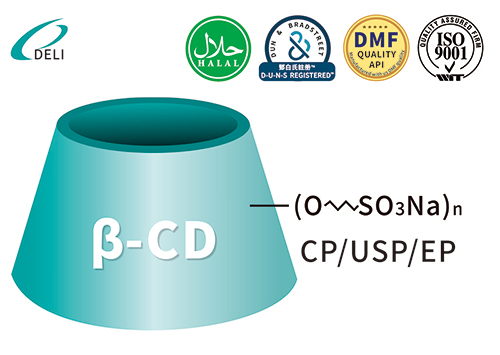 Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae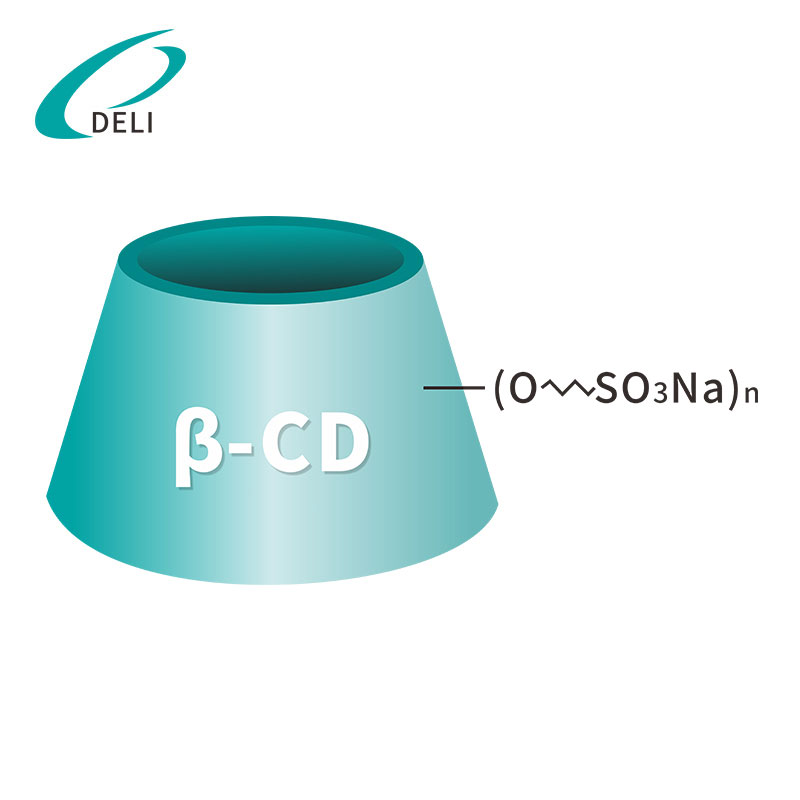 Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0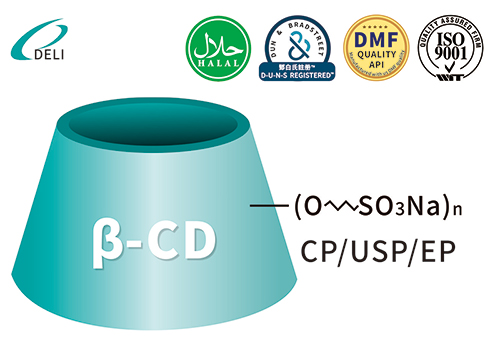 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0