
Betadex Sulfobutyl Ether Sodium pulveris CAS 182410-00-0
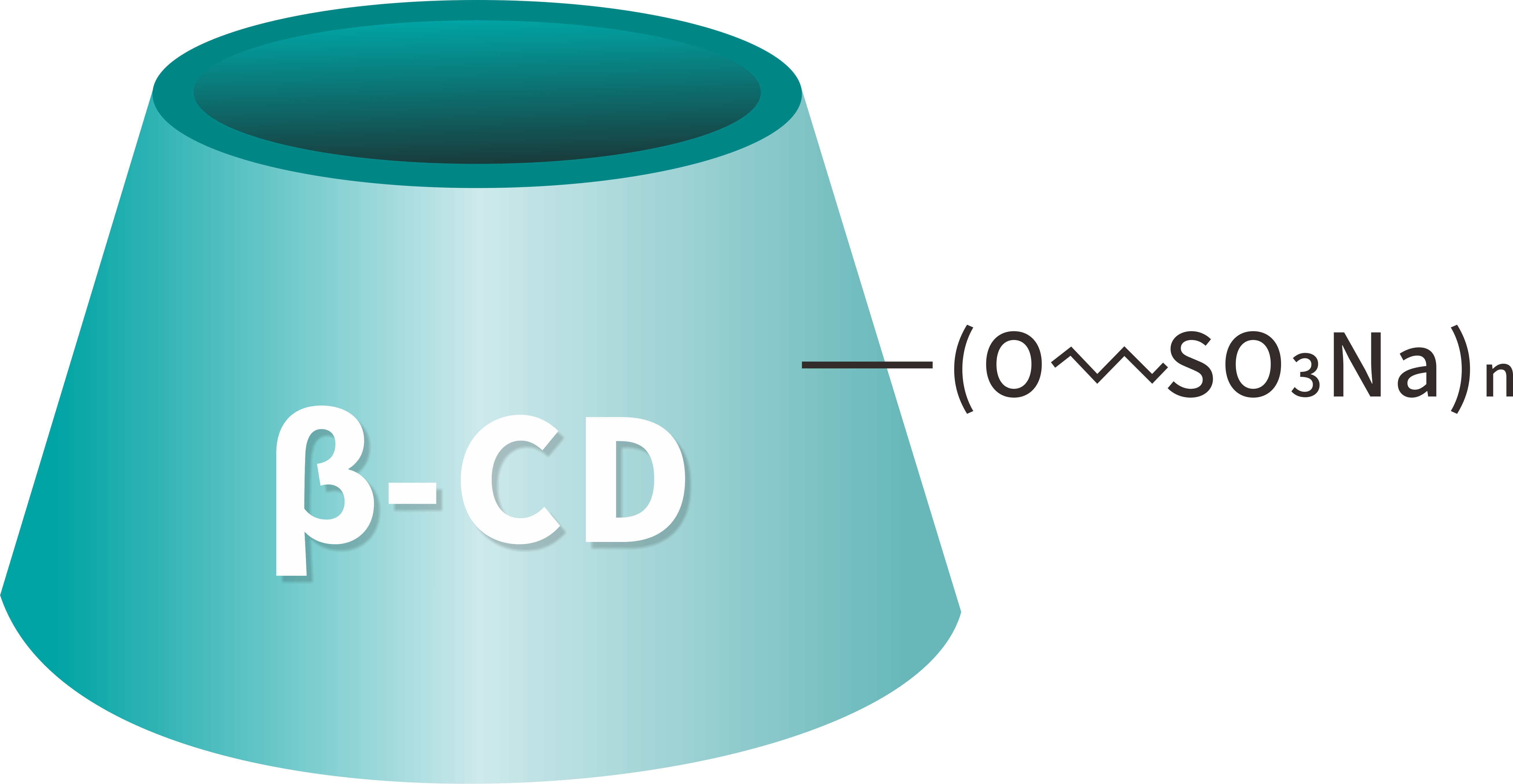
Formulae hypotheticae: C42H70-nO35•(C4H8SO3Na)n
Specification: 500g/Bag; 1kg/Bag; 10kg/bag/orum; aut ut postulationem tuam
Categoria: Exceptiones pharmaceuticae
Repono: Sigillum reservatum.
Tempus validitatis: 36 months
Betadex Sulfobutyl Ether Sodium Powder, etiam quae Sulfobutylether Beta Sodium Cyclodextrin (SBECD), est valde aqua-solubilis, anionica aba cyclodextrin derivativa late adhibita ut exceptam pharmaceuticam injectabilem. Cum CAS numero 182410-00-0, hoc productum speciatim designatum est ad augendam solutionem, stabilitatem, et incolumitatem de rebus pharmaceuticis male aquaticis solubilibus (APIs).
Per formationem complexorum stabilium, non-covalentium inclusionum, Betadex Sulfobutyl Ether Sodium Pulvis efficacem praebet formulam provocandi APIs quae aliter difficilia sunt ad itinera per parenteralia liberanda. Praeclara eius biocompatibilitas et humilis toxicity profile eam apprime aptam reddunt ad administrationem intravenosam repetitam.
Betadex Sulfobutyl Ether Sodium Pulvis producitur a chemica modificatione beta cyclodextrin cum sulfobutylo aethereorum sub conditionibus alcalinis moderatis, sequitur neutralizationem ut sal sodium formet. Haec modificatio hypothetica insigniter melioratur solubilitatem aqueam cum indigenis beta cyclodextrin comparatam, validam complexionem servans facultatem cum moleculis medicamento hydrophobicis.
Cavum hydrophobici structurae cyclodextrinis encapsulat APIs lipophilica, dum hydrophilica exterioris superficiei optimam in aqua solubilitatem efficit. Quam ob rem pharmaca solubilitas, bioavailability et formula stabilitatis valde emendantur sine necessitate durae solutionis organicae vel surfactants.
Betadex Sulfobutyl Ether Sodium Pulvis imprimis ad formulas medicamentorum injectabiles et parentales adhibentur. Late adhibitum est in medicamentis antifungalis, antiviralibus, oncologia, cardiovascularibus, praesertim ubi magna vis medicamentorum et aquarum pauperum solubilitas praesentem formulam provocat.
Haec excepta feliciter adhibita est in productis injectis venalibus, ut voriconazolum, posaconazolum, remdesivir, et alia medicamenta cura critica, demonstrans eius probatam observantiam clinicam et regulatoriam acceptationem.
Product nomen:Betadex Sulfobutyl Ether Sodium Powder
CAS No.:182410-00-0
Aspectus:Alba ad album, amorpho, hygroscopic pulveris
Gradus:Injectable pharmaceutica excepta
Signa:USP/EP/ChP
Pertentare:95.0% - 105.0% (anhydrous basis)
Bacterial Endotoxins:≤ 10 EU/g
Mediocris Substitutionis gradus:Imperium consistentque batch ad batch

Xi'an Deli Industry Biochemical Co., Ltd. est fabrica professionalis specialiter in derivationibus cyclodextrin et cyclodextrin. In MCMXCIX constituta, Deli Biochemical plus XXV annos experientiae deditae in investigationibus pharmaceuticis excipienti, evolutionis et productionis industriae deditae sunt.
Nos in duos nucleos productos intendunt: Hydroxypropyl Betadex (HPBCD) et Betadex Sulfobutyl Ether Sodium (SBECD). Cum technicis cumulus et continuus optimizationis processus diu-terminus, fructus nostri pharmaceuticae clientes ubique terrarum praebentur pro fabricandis evolutionibus clinicis et commercialibus.
Facultates productionis nostrae ordinantur ad requisita fabricandi pharmaceutica occurrentes, inter regiones mundas moderata et processum strictum imperium. Magna-scalae lineae productionis operantur cum capacitate annua circiter 200 talentorum metricorum Betadex Sulfobutyl Ether Sodium, stabilis praestandi copiam cooperationis diuturnae.

Qualitas nostra media est in fabricandis philosophicis. Quaelibet massa Betadex Sulfobutyl Ether Sodium Pulvis subit qualitatem comprehensivam probatio, inclusa analysi puritatis, immunditiae profilingae, endotoxin temperantiae, gradum substitutionis verificationis.
Nostra ratio qualitatis administrandi ratio ISO 9001 signis certificata est, et instrumentis analyticis provectis ut HPLC, IC, GC, et electrophoresis capillaribus sustentatur. Testimonia plena Analysis (COA) omnique sit amet parata ad obsequium delineabilitatis et regulatorii curandum.

Cum decenniis experientiae in fabricandis cyclodextrinis, Xi'an Deli Biochemical plus quam iustum productum praebet. Certam qualitatem, stabilem copiam et technicam professionem sustentationem in vita activitate praestamus. Experientia nostra cum global moderantibus requisitis nobis concedit clientes in diversis mercatis fiducialiter sustentare.
Pro directo opifice, massam qualitatem convenientem, copiam competitive facultatem, et diuturnum cooperationem pro sociis pharmaceuticis quaerunt, ex Galeno injectabilem injectabilem suppeditant.

Ad exempla, documenta technica, seu inquisitiones commerciales circaBetadex Sulfobutyl Ether Sodium pulveris CAS182410-00-0, placere contactus Xi'an Deli Deli Biochemical Industry Co., Ltd. Manipulus noster libenter tuam formulam et necessitates suppeditabit.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0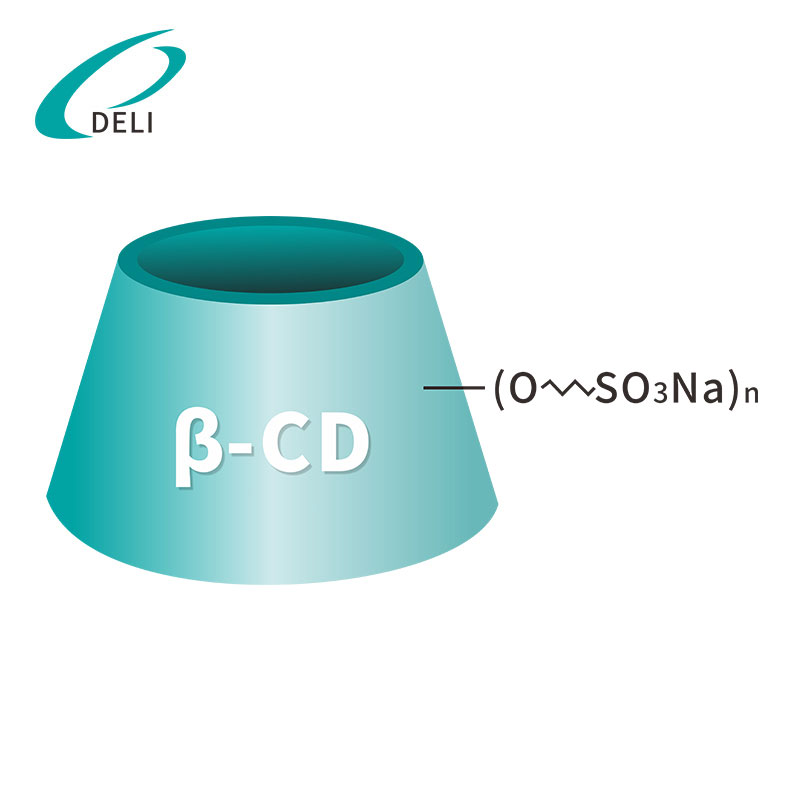 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0