
Iniectio Grade Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Product Name: Betadex Sulfobutyl Ether Sodium
CAS No.: 182410-00-0
Gradus: Iniectio Grade
Standard: USP / EP / ChP
Application: Pharmaceutical Excipient
Aspectus: Alba ad extemporalitatem album, amorpho pulveris
Solubilitas: Gratis solutum in aqua
Packaging: 500 g/peram, 1 kg/peram, 10 kg/orum; nativus packaging praesto
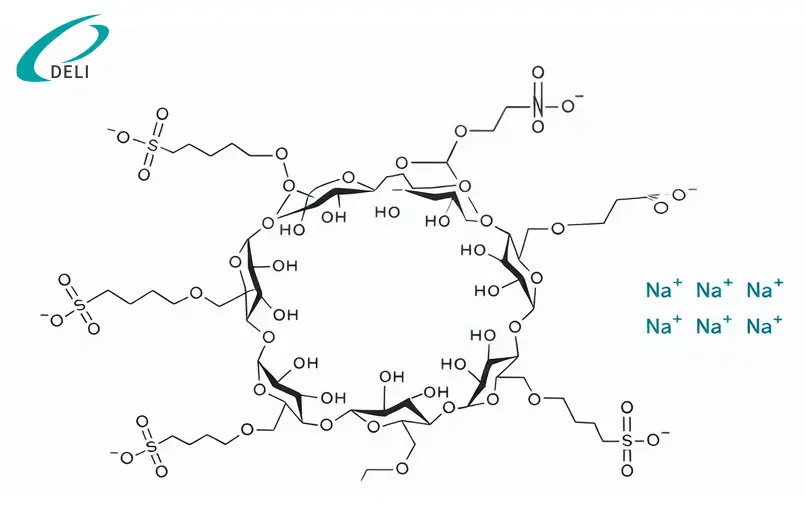
Productum formae firmumnon-covalent inclusione complexorumcum rebus pharmaceuticis activis, praesertim elementis fundamentalibus et nitrogenio-continentibus. Hoc commercium medicamento solubilitatem ac formulationem perficiendi sine chemico modificatione API meliorem meliorem facit.
Betadex Sulfobutyl Ether Sodium Iniectio Gradus principaliter adhibetur in formis parentum et aliis dosis non-oralis et agnoscitur pro sua prosperitate profile cum patria beta-cyclodextrin comparata.
Product nomen:Betadex Sulfobutyl Ether Sodium
CAS No.:182410-00-0
Gradus:Iniectio Grade
Latin:USP/EP/ChP
Applicatio:Pharmaceutical Excipient
Aspectus:Alba ad extemporalitatem album, amorpho pulveris
Solubilitas:Solutum in aqua
Packaging:500 g/pera, 1 kg/pera, 10 kg/orum; nativus packaging praesto
Productum fabricatum est sub stricta qualitate administrationis systematis velandi materiam materiae rudis, processus sanationis, et operis effecti probationem. Clavis qualitas attribuit ut identificatio, primordium, profanum immunditia, endotoxins bacterial, limites microbiales, gradus substitutionis moderantur secundum requisita pharmacopeiae.
Xi'an DELI Industry Biochemical Co., Ltd. tenet integram corporatum et qualitatem productionis, inclusa licentias negotiationes validas, productio pharmaceutica permittit, HALAL certificationem, DMF sustentationem, aliaque documenta moderantia spectantia. Supportantes technicae et obsequia documenta praesto sunt postulantibus.

Iniectio Grade Betadex Sulfobutyl Ether Sodium imprimis applicatur in formis dosis pharmaceuticae non oralis, ubi auctae sunt solutionibilitas, stabilitas et salus. In solutionibus injectabilibus late adhibetur ad solutionem aqueam solutionis male aquee-solubilis medicamentis male pharmaceuticae activum, et in repositione et administratione formulam claritatis ac stabilitatis conservare.
In pulveribus lyophilisatis pro iniectione, productus sustinet complexiones inclusionum stabilium formationes, adiuvans ad conservationem APIs sensitivarum in processibus congelatis siccitatis et restitutionis. Praeclara eius compatibilitas idoneos facit ad formulas quae celeri dissoluuntur et congruenter perficiuntur post restitutionem.
Productum etiam applicatur in formulis ophthalmicis, ubi humilis toxicitas, summa puritas, tolerabilitas bona critica sunt. Medicamento solubilitatem emendans et irritationem potentialem reducendo, ad guttam solutionum oculi lucidi et stabilis confert. In praeparatione nasi medicamento solutionem auget et sustinet partum efficientem per mucosae nasi servata formula salutis.
Praeterea Iniectio Gradus Betadex Sulfobutyl Ether Sodium apta est pro inspiratione formularum quae sequuntur formulam aestimationem aptam. Facultas eius complexiones inclusion convertendi formandi et necessitatem durarum solubilium minuendi facit eam pretiosam excipientem ad systemata non-oralis medicamentorum partus provectae.

Productum praestans aquae solubilitatem et validam solutionem capacitatis praebet ad medicamenta pharmaceutica male aqua-solubilia activa, id quod maxime convenit ad formulas injectabiles complexas. Per formationem complexorum stabilium et convertibilium non covalentium, signanter auget stabilitatem formulam, servata originalis structurae chemicae substantiae medicamento.
Comparatus cum indigenis beta-cyclodextrinis et solubiliariis quibusdam conventionalibus, Betadex Sulfobutyl Ether Sodium laetiorem profile salutem exhibet, cum actione hemolytica reducta et toxicity renalis inferior, quae usum in parenteralibus et aliis sensitivis dosis formis sustinet. Eius structura anionica ad meliorem convenientiam confert cum APIs amplis, compositionibus praesertim nitrogenio-continentibus.
Praeterea producti formulam evolutionis flexibilem adiuvat meliore medicamento bioavailability, necessitatem solvendi organici solvendi, et solutiones aqueas claras et stabiles expediet. Hoc facit praelatum exceptam electionem pro modernis iniectibilibus, ophthalmicis, et systematibus medicamentorum partus provectis.
Xi'an DELI Industry Biochemical Co., Ltd. plus 26 annos experientiae habet in evolutione et m.de cyclodextrinis earumque derivatis anufactura. Diu terminus noster focus in excipientibus pharmaceuticis permittit ut fructus emittat cum qualitate constanti, certa operatione, et stabili copia pro clientibus globali.
Facilitas productionis dedicatae operamur, quae stricta quali- tate administrandi ratio sustinetur, quae materias rudis, in processu temperantiae et molis probationis operit. Hoc efficit batch-ad-batch constantiam et obsequium cum requisitis pharmacopeiae ad applicationes pharmaceuticae et non-orales.
Noster Betadex Sulfobutyl Ether Sodium Iniectio Gradus fabricatur cum substitutione moderatae et puritatis altae, dum certam solutionem et formulam stabilitatem APIs male aquatilis solubilis praebeat. Productum constituitur obviam technicis et moderantibus exspectationibus pharmaceuticae formulae evolutionis.
DELI Biochemicus tenet corporatum et industriam fabricandi, inter valida licentias negotiationes, productio pharmaceutica permittit, HALAL certificationem et DMF sustentationem. Documenta technica comprehensiva et subsidia moderantia praeberi possunt ad auxilium productum adnotatione et diuturnum cooperationem.
Cum capacitate productionis stabilis, auxilio technico professionali, et communicatione responsiva, committendum est ut societates diuturnae aedificandae secundum qualitatem, fidem et fiduciam.


Q: Quid est Iniectio Grade Betadex Sulfobutyl Ether Sodium usus for
A: Ex Galeno pharmaceutico usus est ad solutionem ac stabilitatem stabilitatemque male aquaticae pharmaceuticae per convertibilem inclusionem complexam formationem ingrediendi activum solubilem emendare.
Q: Hoc productum est apta formulae ad parenterale
A: Ita. Iniectio Gradus Betadex Sulfobutyl Ether Sodium nominatim ad applicationes pharmaceuticae parenterale destinatur, inter solutiones injectabiles et pulveris lyophilizati pro iniectione.
Q: Hoc productum chemica mutare API
A: N. Inclusionem complexorum cum APIs non-covalente format, et structuram chemicam substantiae medicamentorum non mutat.
Q: Quod formas dosis hoc productum potest esse in
A: Maxime adhibetur in formis dosis non-oris sicut solutiones injectabiles, injectiones duratae, formulae ophthalmicae, praeparationes nasi et inspirationes formulae, subiectae formulae aestimationee.
Q: Quomodo hoc productum diversum ab indigena beta cyclodextrin?
A: Comparatus cum cyclodextrino beta nativo, Betadex Sulfobutyl Ether Sodium praebet aquam solubilitatem altiorem et profile salutem commodiorem, aptam ad usum injectabilem faciens.
Q: productum est obsecundantia signa pharmacopeiae
A: Productum sub districta qualitatum potestate conficitur et pharmacopeiae requisitis obtemperat ut USP et EP secundum specificationes internas.
Q: sunt regulatory et technica documenta praesto
A: Ita. Documenta technica, materias qualitates relatas, et DMF firmamentum roganti praeberi possunt, ut evolutionis formulae et producti adnotationem sustineant.
Q: Utrum opificem longum tempus et stabilem copiam praebeat?
A: Ita. Xi'an DELI Industry Biochemical Co., Ltd. dedicavit facultatem productionis faciendi facultatem stabilem et congruentem batch-ad-batch qualitatis.
 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0