
DELI isBetadex Sulfobutyl Ether Sodium USP 99% Iniectio Gradus opificem. Productum nostrum DMF numerus est 034773. Systema administrationis societatis ISO 9001:2015 certificatione transiit.
[Latin]: EP/USP
[MOQ]: 500g
[Molecular formula]: C42H70-nO35•(C4H8SO3Na)n[Tempus validitatis]: 36 months
[β-Cyclodextrin Content]: NMT 0.1%
Integer non libero lacus.
1. Rescribe inquisitioni tuae prompte ac bona cito libera.
2. Societas producti Hydroxypropyl Betadex qualitatem certitudinis et copiae stabilis, occurrit norma postulatorum ChP, USP et EP. Betadex Sulfobutyl Ether Sodium Iniectio Gradus occurrit normae postulatis USP et EP.
3. Lorem products ut peculiari postulatione tua possumus.
4. Betadex Sulfobutyl Ether Sodium Iniectio Gradus emitur in Asiam, Europam, Americam Septentrionalem, Africam et alias regiones.


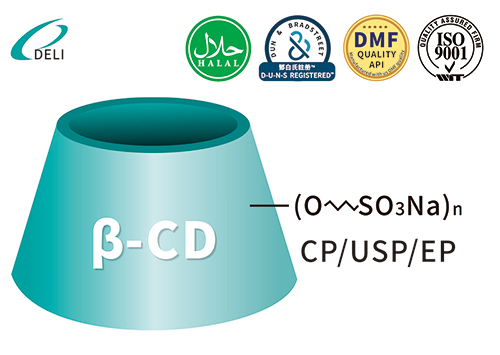 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0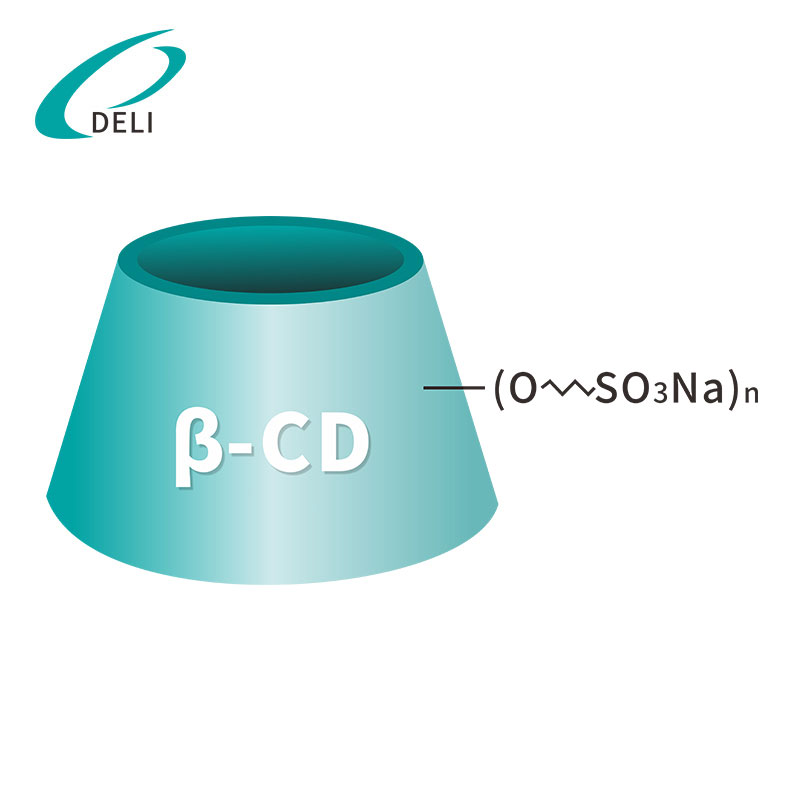 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium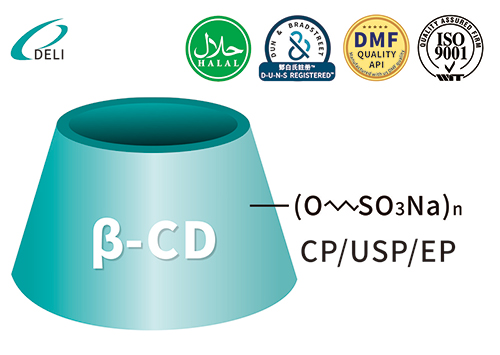 Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade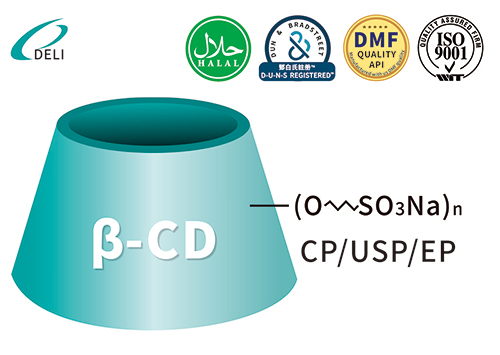 Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae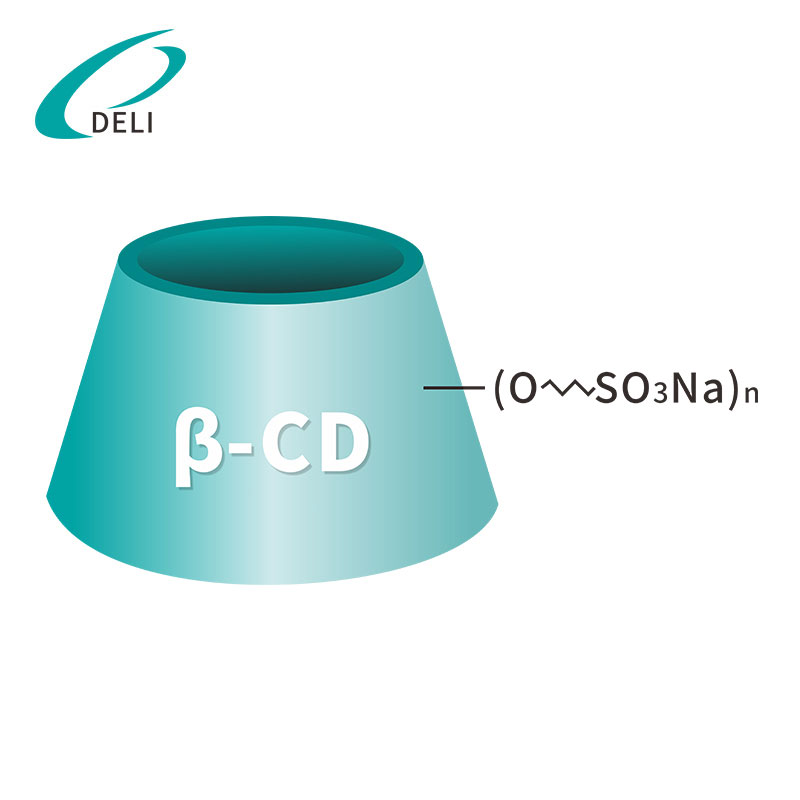 Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0