


Sulfobutyl Beta cyclodextrin Sodium CAS 182410-00-0 est cyclodextrinus aquaticus solubilis admodum exceptus derivativus pharmaceuticus. Hic gradus injectabilis SBECD solubilitatem, stabilitatem, et incolumitatem APIs male solutorum in formulis parenteralibus et oralibus meliorat.
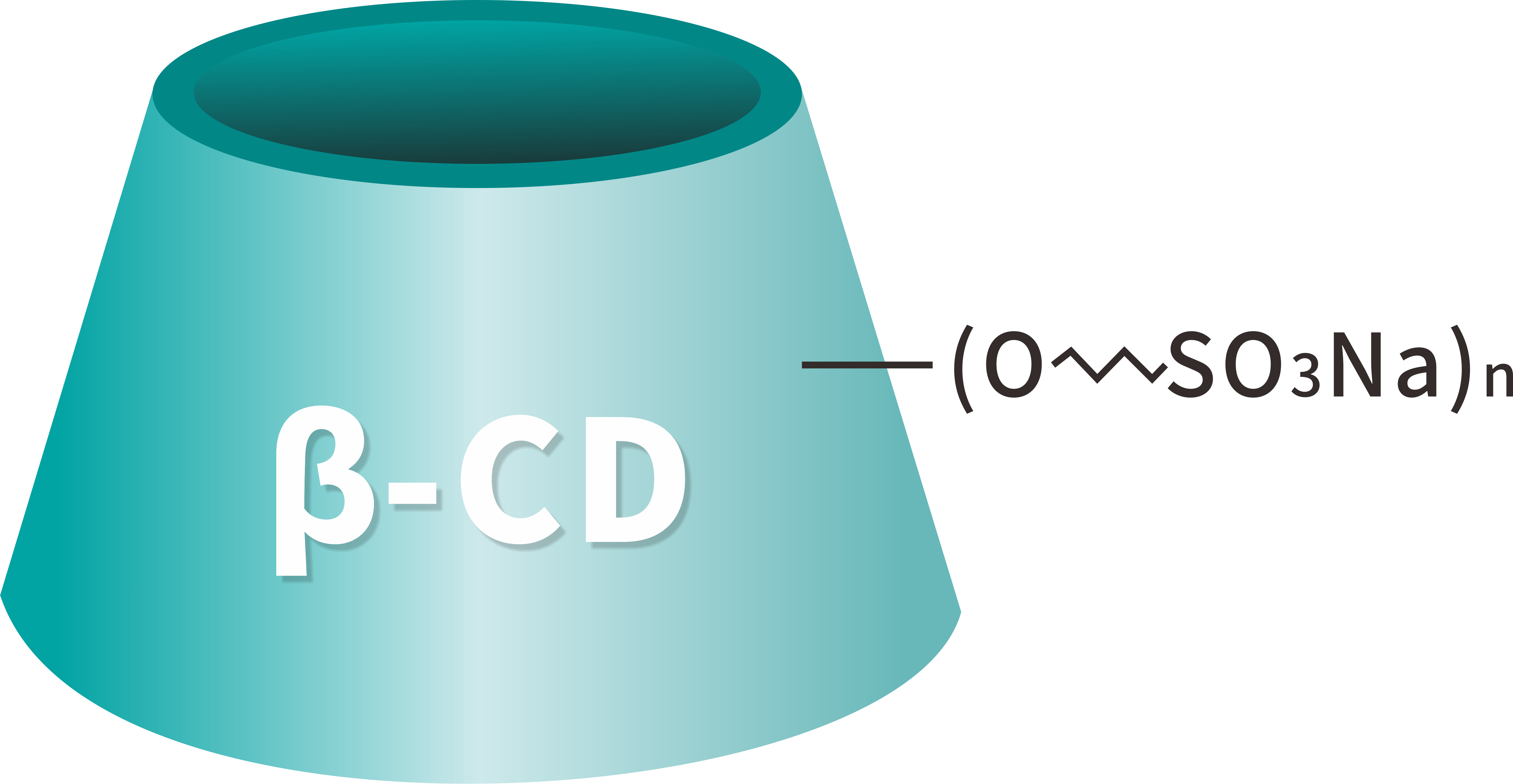
Sulfobutyl Beta Cyclodextrin Sodium CAS 182410-00-0, also known asSulfobutyl Beta Sodium Cyclodextrin (SBECD)Est admodum solutum aquarum cyclodextrinum anionicum derivativum pro applicationibus pharmaceuticis exceptam evolutam. Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0 late pro solubilizer adhibetur, udus agente, agente chelating (agente implicato), et agente in polyvalent masking medicamento formulae provectus.
Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0 feliciter applicatum est injectabiles, orales, nasales, medicamenta medicamentorum ophthalmicorum. Sulfobutyl Beta Cyclodextrin Sodium capacitatem inclusionem validam ostendit et affinitatem specificam ad medicamenta NITROGENIUM continens; pharmacum efficaciter emendans solubilitatem, stabilitatem et formulam salutis per non-covalentes complexus.
In medicamentis medicamentis, Betadex Sulfobutyl Ether Sodium CAS N. 182410-00-0 adiuvat toxicitatem renum minuere, hemolysin minuere, pharmacum emissio control rates, larva injucunda odores, Sulfobutyl Beta Cyclodextrin Sodium maxime idoneum ad parenteral formulae requirunt altam salutem et observantiam.
[CAS No.]: 182410-00-0
[Formula hypothetica]: C42H70-nO35·(C4H8SO3Na)n
[Gradus]: Injectable / Pharmaceutical Grade
[Executive Standard]: USP / EP / Enterprise Standard
[specification]: 500 g/peram; 1 kg/bag; 10 kg/peram; 10 kg/drum
[Applicatio Area]: Ex Galeno pharmaceutico
| Descriptio et Solubility | Alba ut off-w*hite amorphous pulveris. Gratis solutum in aqua; parce solutum in methanolum; fere insolubilem in ethanolo, n-hexane, 1-butanolo, acetonitrile; 2-propanol, et acetatis ethyl. |
| Lepidium sativum A (IR), USP <197K> | Prosequitur SBECD reference vexillum |
| identificatio B (HPLC), USP <621> | Retention tempus maioris apicem prosequitur SBECD reference |
| Lepidium sativum C (CE), USP <1053> | Meets requisitis Mediocris Substitutionis gradus |
| Lepidium sativum D, USP <191> | Positive reactionem for sodium |
| (HPLC), USP <621> | 95.0% - 105.0% (anhydrous basis) |
| Finis Betadex | NMT 0.1% |
| Terminus 1,4-Butane Sultone | NMT 0.5 ppm |
| Terminus Sodium Chloride | NMT 0.2% |
| Bacterial Endotoxins, USP <85> | ≤10 EU/g |
| Limites Microbiales, USP <61> |
TAMC ≤ 100 cfu/g TYMC ≤ 50 cfu/g |
| Certa Microorganismi, USP <62> | De absentia Escherichia coli in 1 g |
| Mediocris gradus substitutionis (DS) | 6.2 - 6.9 |
Potentialis pharmaceuticae derivationum beta-cyclodextrin per confirmatum est decades investigationis. Comparatur cum indigena beta cyclodextrin.Betadex Sulfobutyl Ether Sodium CAS 182410-00-0monstrat melius salus et solubilis effectus, faciens Sulfobutyl Beta Cyclodextrin Sodium an efficax excepta ad bioavailability augendi medicamentorum male solutorum, praesertim BCS Classis II componit.
Xi'an Deli Industry Biochemical Co., Ltd. plenum technicum subsidium praebet, inter COA, MSDS, formula auxilii. Sample iudicium et nativus packaging solutiones praesto sunt ad petitionem.
Formulae progressionis, documentorum moderantium, vel mercatorum copia inquisitionum ad Betadex Sulfobutyl Ether Sodium CAS No. 182410-00-0, pete contactum Xi'an Deli Nostrae technicae turmae subsidia incepta ab R&D ad scalam commercialem sustinet.


 Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS NO 182410-00-0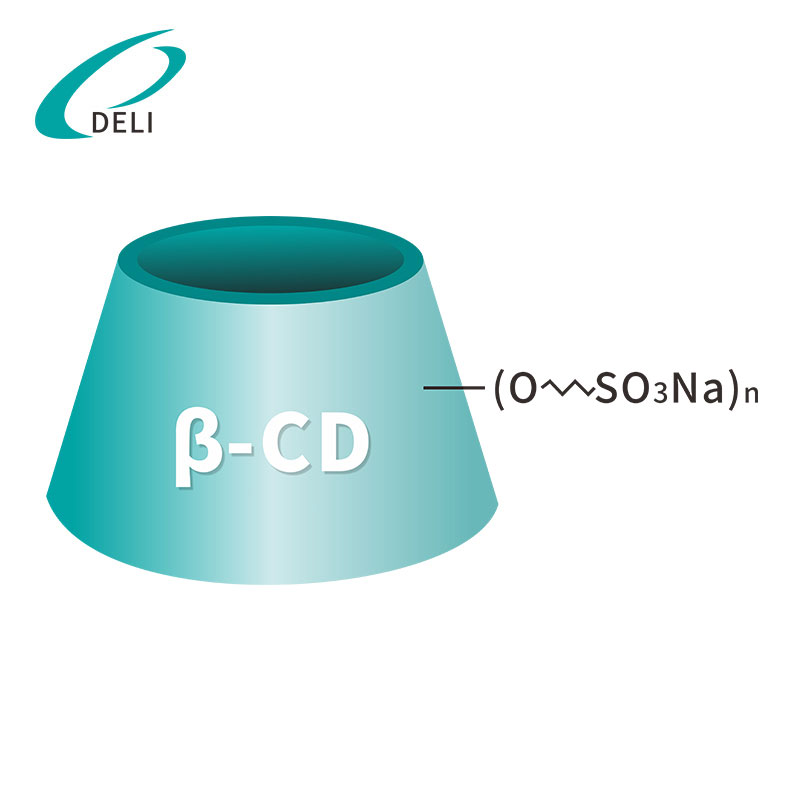 Sulfobutyl Ether Beta Cyclodextrin Sodium
Sulfobutyl Ether Beta Cyclodextrin Sodium Betadex Sulfobutyl Ether Sodium Iniectio Grade
Betadex Sulfobutyl Ether Sodium Iniectio Grade Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae
Betadex Sulfobutyl Ether Sodium SBECD Injectable Gradus ad medicamentis formulae Betadex Sulfobutyl Ether Sodium CAS 182410-00-0
Betadex Sulfobutyl Ether Sodium CAS 182410-00-0 DMF Betadex Sulfobutyl Ether Sodium 182410-00-0
DMF Betadex Sulfobutyl Ether Sodium 182410-00-0